ケミカルバイオロジー研究グループ(終了)
4-Isoavenaciolide
- Structure
-

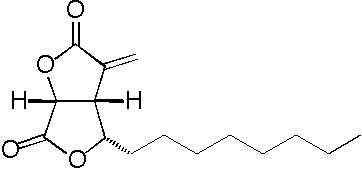
- Producing organism
- Aspergillus sp.
- Biological activity
- dual specificity phosphatase inhibitor
- Abstract
- A potent inhibitor of a dual-specificity protein phosphatase, VHR (vaccinia H1 related), was isolated during a screening of microbial metabolites. This inhibitor was identified as 4-isoavenaciolide (4-iA), and was determined to irreversibly inhibit VHR phosphatase activity with a 50% inhibitory concentration of 1.2 µM. Detailed tandem mass spectrometry analyses of proteolysed fragments revealed that two molecules of 4-iA bound a molecule of VHR at the two different fragments: one containing the catalytic domain and the other containing the α6 helix positioned surface domain. As 4-iA possesses a reactive exo-methylene moiety, it is possible that 4-iA inhibits VHR through the direct binding to the cysteine residue in the catalytic site (Cys124). Furthermore, 4-iA inhibited dual-specificity protein phosphatases and tyrosine phosphatases, but did not inhibit serine/threonine phosphatases. These results suggest that 4-iA is a cysteine-targeting inhibitor of protein phosphatases with a common HCX5RS/T motif in the catalytic site.
- References
-
isolation and structural determination
- Brookes D, Tidd BK, Turner WB.
Avenaciolide, an antifungal lactone from Aspergillus avenaceus.
J. Chem. Soc., 5385-5391 (1963) [ doi: 10.1039/JR9630005385 ]biological activity- Ueda K, Usui T, Nakayama H, Ueki M, Takio K, Ubukata M, Osada H.: 4-isoavenaciolide covalently binds and inhibits VHR, a dual-specificity phosphatase.
FEBS Lett, 525(1-3): 48-52 (2002)
- Brookes D, Tidd BK, Turner WB.


