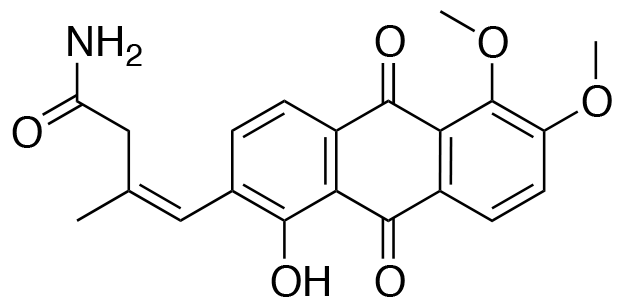
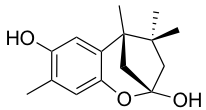
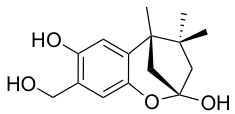
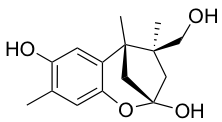
YO-001A
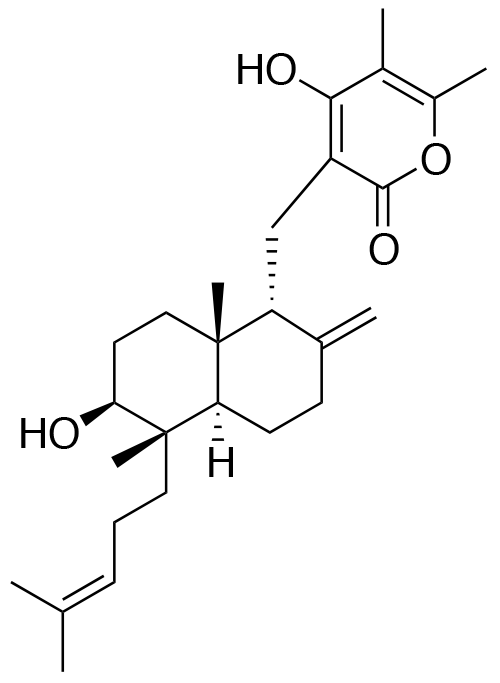
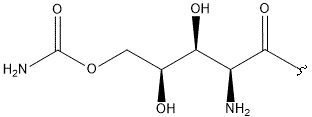
- Structure
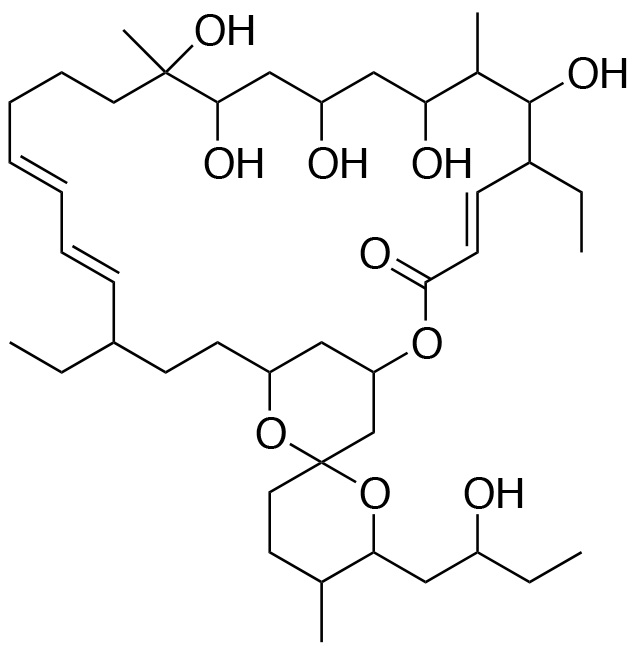
-

- Producing organism
- Streptomyces sp. YO15-A001
- Biological activity
- antifungal, inhibition of the FOF1-ATPase activity
- Abstract
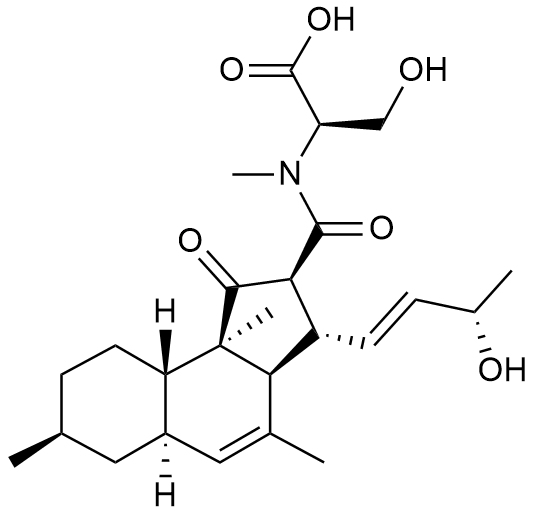
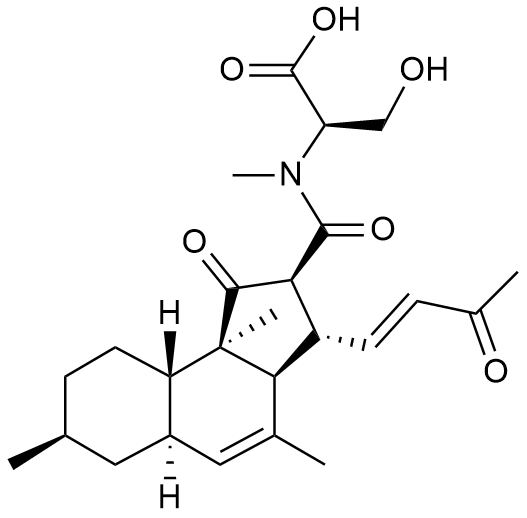
- A new antifungal compound YO-001A was found from the culture broth of Streptomyces sp. YO15-A001, which was isolated from a soil sample collected in Toyama Prefecture. YO-001A was identified through morphological changes-based screening of the rice blast fungus, Pyricularia oryzae (P. oryzae). YO-001A is a new 26-membered macrolide of the oligomycin family, which exhibits potent antifungal activity against P. oryzae with an IC50 of 0.012 µM by disrupting mitochondrial respiration via inhibition of the FOF1-ATPase activity.
- References
-
- Yamamoto K, Futamura Y, Uson-Lopez RA, Aono H, Shimizu T, Osada H.: YO-001A, a new antifungal agent produced by Streptomyces sp. YO15-A001.
J Antibiot, 72, 986-990 (2019) [ doi: 10.1038/s41429-019-0239-z ]
[ doi: 10.1038/s41429-019-0239-z ]
- Yamamoto K, Futamura Y, Uson-Lopez RA, Aono H, Shimizu T, Osada H.: YO-001A, a new antifungal agent produced by Streptomyces sp. YO15-A001.