ケミカルバイオロジー研究グループ(終了)
Stevastelin
- Structure
-
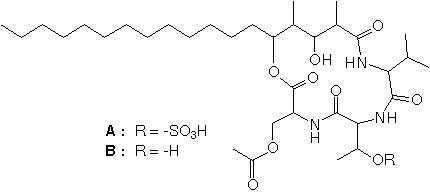
- Producing organism
- Penicillium sp.
- Biological activity
- dual-specificity protein phosphatases inhibitor
- Abstract
- Since the molecular target of the immunosuppressive reagents FK506 and cyclosporin A was revealed to be protein phosphatase PP2B (calcineurin), many researchers have been screening the protein phosphatase inhibitors from microbial metabolites to develop new immunosuppressive reagents. We isolated stevastelin B, which is composed of valine, threonine, serine and 3,5-dihydroxy-2,4-dimethyl stearic acid, and stevastelin A, which is a sulphonylated derivative of stevastelin B. To understand the action mechanism of stevastelins A and B, we synthesized a series of stevastelin derivatives and investigated their structure-activity relationships. RESULTS: A series of stevastelin derivatives have been systematically synthesized. Stevastelin B inhibited gene expression that is dependent on interleukin-2 (IL-2) or IL-6 promoters in situ, but it had no inhibitory activity against any protein phosphatases in vitro. In contrast, stevastelin A, which is a sulphonylated derivative of stevastelin B, inhibited the phosphatase activity of a dual-specificity phosphatase, VH1-related human protein (VHR), in vitro, but it had no inhibitory activity against gene expression or cell-cycle progression in situ. CONCLUSIONS: Stevastelin B is a novel immunosuppressant. It inhibited IL-2 or IL-6 dependent gene expression but did not inhibit the phosphatase activity of calcineurin. The structure-activity relationships show that the acidic functional group on the threonine residue and the stearic acid moiety in the stevastelin molecule are important for inhibitory effects on the dephosphorylation activity of VHR in vitro. Stevastelin B might be sulphonylated or phosphorylated after incorporation into the target cell, and then it interacts with protein tyrosine phosphatases and regulates cell-cycle progression.
- References
-
isolation and structural determination
- Morino T, Shimada K, Masuda A, Yamashita N, Nishimoto M, Nishikiori T, Saito S.: Structural determination of stevastelins, novel depsipeptides from Penicillium sp.
J Antibiot, 49(6): 564-568 (1996)
- Shimada K, Morino T, Masuda A, Sato M, Kitagawa M, Saito S.: Absolute structural determination of stevastelin B.
J Antibiot, 49(6): 569-574 (1996)
- Morino T, Shimada K, Masuda A, Nishimoto M, Saito S.: Stevastelin A3, D3 and E3, novel congeners from a high producing mutant of Penicillium sp.
J Antibiot, 49(10): 1049-1051 (1996) biological activity
biological activity- Hamaguchi T, Masuda A, Morino T, Osada H.: Stevastelins, a novel group of immunosuppressants, inhibit dual-specificity protein phosphatases.
Chem Biol, 4(4): 279-286 (1997)
- Morino T, Shimada K, Masuda A, Yamashita N, Nishimoto M, Nishikiori T, Saito S.: Structural determination of stevastelins, novel depsipeptides from Penicillium sp.


