ケミカルバイオロジー研究グループ(終了)
Juniferdin
- Structure
-

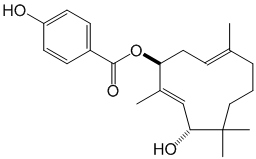
- Producing organism
- Ferula junipernia
- Biological activity
- protein disulfide isomerase inhibitor
- Abstract
- Protein disulfide isomerase (PDI) is a promiscuous protein with multifunctional properties. PDI mediates proper protein folding by oxidation or isomerization and disrupts disulfide bonds by reduction. The entry of HIV-1 into cells is facilitated by the PDI-catalyzed reductive cleavage of disulfide bonds in gp120. PDI is regarded as a potential drug target because of its reduction activity. We screened a chemical library of natural products for PDI-specific inhibitors in a high-throughput fashion and identified the natural compound juniferdin as the most potent inhibitor of PDI. Derivatives of juniferdin were synthesized, with compound 13 showing inhibitory activities comparable to those of juniferdin but reduced cytotoxicity. Both juniferdin and compound 13 inhibited PDI reductase activity in a dose-dependent manner, with IC50 values of 156 and 167 nM, respectively. Our results also indicated that juniferdin and compound 13 exert their inhibitory activities specifically on PDI but do not significantly inhibit homologues of this protein family. Moreover, we found that both compounds can inhibit PDI-mediated reduction of HIV-1 envelope glycoprotein gp120.
- References
-
- Khan MM, Simizu S, Lai NS, Kawatani M, Shimizu T, Osada H.: Discovery of a small molecule PDI inhibitor that inhibits reduction of HIV-1 envelope glycoprotein gp120.
ACS Chem Biol, 6(3): 245-251 (2011) [ doi: 10.1021/cb100387r ]
[ doi: 10.1021/cb100387r ]- Khan MM, Simizu S, Kawatani M, Osada H.: The potential of protein disulfide isomerase as a therapeutic drug target.
Oncol Res, 19(10-11): 445-453 (2011)
- Khan MM, Simizu S, Lai NS, Kawatani M, Shimizu T, Osada H.: Discovery of a small molecule PDI inhibitor that inhibits reduction of HIV-1 envelope glycoprotein gp120.


