Screening for PBD-dependent binding inhibitors.
Outlines:
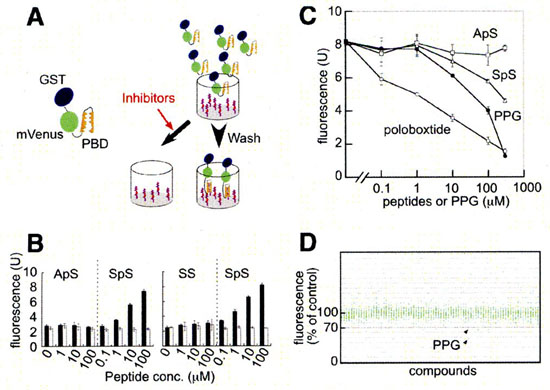
Plk1 possesses a phosphopeptide-binding domain named Polo box domain (PBD) at its C-terminus. PBD is postulated to be essential for Plk1 localization and substrate targeting. We developed a high-throughput screening system to identify inhibitors of PBD-dependent binding and screened a chemical library.
Phosphopeptides (100 uL/well of 10 uM solution, otherwise noted) were covalently bound to maleimide-activated 96-well plates (PIERCE) as instructed by the supplier. GST-mVenus-PBD and its mutant were expressed in E. coli BL21 (DE3). The bacteria were lysed by sonication in sonication buffer (20 mM Tris, pH 8.0/125 mM NaCl/0.5% Nonidet P-40/1 mM EDTA/1 mM DTT/200 uM Na3VO4/50 mM NaF) supplemented with Complete protease inhibitors (Roche Applied Science) and 0.1% lysozyme. After centrifugation, the bacterial lysate was mixed with candidate compounds (at 50 M in the first screening) and put into wells of the phosphopeptide-bound plate and incubated at 4°C overnight. Bound GST-mVenus-PBD was quantitated by spectrofluorometry (ex: 485 nm, em: 530 nm) after five rinses with 0.05% Nonidet P-40 in PBS and two rinses with PBS.
 |
|
Screening system for inhibitors of PBD-dependent binding and identification of PPG as an inhibitor of PBD-dependent binding. |
References:
-
N. Watanabe, T. Sekine, M. Takagi, J.I. Iwasaki, N. Imamoto, H. Kawasaki,H. Osada.
Deficiency in chromosome congression by the inhibition of PLK1 polo box domain-dependent recognition.
J. Bio. Chem., 284, 2344-2353 (2009).


