Unantimycin A
- Structure
-

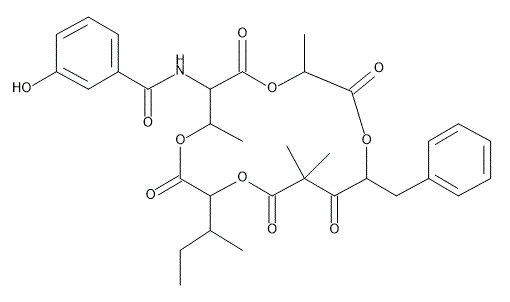
- Producing organism
- Streptomyces sp. RK88-1355
- Biological activity
- cytotoxicity and antimalarial activity
- Abstract
- In the course of our screening for structurally unique secondary metabolites from a microbial metabolite fraction library by spectral database search, a new neoantimycin analog, unantimycin A was discovered and isolated. The structure was determined based on extensive spectroscopic methods, including NMR and MS. It had a 3-hydroxybenzoic acid moiety instead of a 2-hydroxy-3- formylaminobenzoic acid moiety, which was a representative functional group for neoantimycin class of metabolites, and showed moderate cytotoxicity against several cancer cell lines.
- References
-
isolation and structural determination
- Lim CL, Nogawa T, Okano A, Futamura Y, Kawatani M, Takahashi S, Ibrahim D, Osada H.: Unantimycin A, a new neoantimycin analog isolated from a microbial metabolite fraction library.
J Antibiot, 69(6): 456-458 (2016) [ doi: 10.1038/ja.2015.124 ]
[ doi: 10.1038/ja.2015.124 ] - Lim CL, Nogawa T, Okano A, Futamura Y, Kawatani M, Takahashi S, Ibrahim D, Osada H.: Unantimycin A, a new neoantimycin analog isolated from a microbial metabolite fraction library.


