Vipirinin
- Structure
-

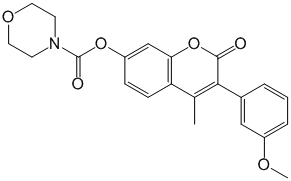
- Producing organism
- (Chemically synthesized)
- Biological activity
- HIV Vpr inhibitor
- Abstract
- The human immunodeficiency virus 1 (HIV-1) viral protein R (Vpr) is an accessory protein that has been shown to have multiple roles in HIV-1 pathogenesis. By screening chemical libraries in the RIKEN Natural Products Depository, we identified a 3-phenyl coumarin-based compound that inhibited the cell cycle arrest activity of Vpr in yeast and Vpr-dependent viral infection of human macrophages. We determined its minimal pharmacophore through a structure-activity relationship study and produced more potent derivatives. We detected direct binding, and by assaying a panel of Vpr mutants, we found the hydrophobic region about residues Glu-25 and Gln-65 to be potentially involved in the binding of the inhibitor. Our findings exposed a targeting site on Vpr and delineated a convenient approach to explore other targeting sites on the protein using small molecule inhibitors as bioprobes.
- References
-
- Ong EB, Watanabe N, Saito A, Futamura Y, Abd El Galil KH, Koito A, Najimudin N, Osada H.: Vipirinin, a coumarin-based HIV-1 Vpr inhibitor, interacts with a hydrophobic region of VPR.
J Biol Chem, 286(16): 14049-14056 (2011) [ doi: 10.1074/jbc.M110.185397 ]
[ doi: 10.1074/jbc.M110.185397 ] - Ong EB, Watanabe N, Saito A, Futamura Y, Abd El Galil KH, Koito A, Najimudin N, Osada H.: Vipirinin, a coumarin-based HIV-1 Vpr inhibitor, interacts with a hydrophobic region of VPR.


