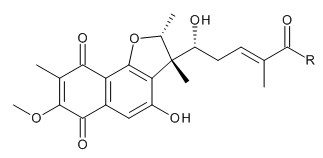
Furaquinocin I, J
- Structure
-
I: R = -OH 
J: R = -NH2 
- Producing organism
- Streptomyces reveromyceticus SN-593
- Biological activity
- (no cytotoxic activity)
- Abstract
- Two novel furaquinocin (FQ) analogues, I (1) and J (2), were isolated from Streptomyces reveromyceticus SN-593 strain NRM2. Their structures were elucidated by MS and NMR analyses. Similar to the previously described FQ D (3), both 1 and 2 possessed a dihydrofuran ring fused to a polyketide naphthoquinone skeleton. The main difference between 1, 2 and 3 was the type of residue attached to C-13; these were a carboxyl, a carboxamide and a methyl residue, respectively.
- References
-
- Panthee S, Takahashi S, Takagi H, Nogawa T, Oowada E, Uramoto M, Osada H.: Furaquinocins I and J: novel polyketide isoprenoid hybrid compounds from Streptomyces reveromyceticus SN-593.
J Antibiot, 64(7): 509-513 (2011) [ doi: 10.1038/ja.2011.41 ]
[ doi: 10.1038/ja.2011.41 ] - Panthee S, Takahashi S, Takagi H, Nogawa T, Oowada E, Uramoto M, Osada H.: Furaquinocins I and J: novel polyketide isoprenoid hybrid compounds from Streptomyces reveromyceticus SN-593.


