Epoxyquinol A, B
- Structure
-
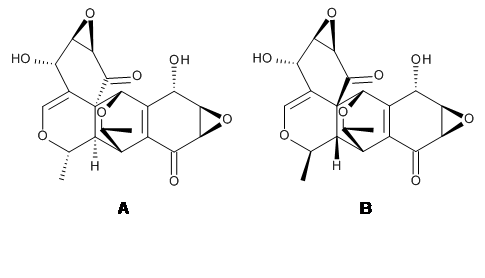
- Producing organism
- uncharacterized fungus
- Biological activity
- antiangiogenic activity
- Abstract
- -----
- References
-
isolation and structural determination
- Kakeya H, Onose R, Koshino H, Yoshida A, Kobayashi K, Kageyama S, Osada H.: Epoxyquinol A, a highly functionalized pentaketide dimer with antiangiogenic activity isolated from fungal metabolites.
J Am Chem Soc, 124(14): 3496-3497 (2002)
- Kakeya H, Onose R, Yoshida A, Koshino H, Osada H.: Epoxyquinol B, a fungal metabolite with a potent antiangiogenic activity.
J Antibiot, 55(9): 829-831 (2002) synthesis
synthesis- Shoji M, Imai H, Mukaida M, Sakai K, Kakeya H, Osada H, Hayashi Y.: Total synthesis of epoxyquinols A, B, and C and epoxytwinol A and the reactivity of a 2H-pyran derivative as the diene component in the Diels-Alder reaction.
J Org Chem, 70(1): 79-91 (2005) [ doi: 10.1021/jo048425h ]
[ doi: 10.1021/jo048425h ]- Shoji M, Yamaguchi J, Kakeya H, Osada H, Hayashi Y.: Total synthesis of (+)-epoxyquinols A and B.
Angew Chem Int Ed Engl, 41(17): 3192-3194 (2002) [ doi: 10.1002/1521-3773(20020902)41:17<3192::AID-ANIE3192>3.0.CO;2-E ]
[ doi: 10.1002/1521-3773(20020902)41:17<3192::AID-ANIE3192>3.0.CO;2-E ]- Shoji M, Kishida S, Takeda M, Kakeya H, Osada H, Hayashi Y.
A practical total synthesis of both enantiomers of epoxyquinols A and B.
Tetrahedron Lett. 43, 9155-9158 (2002) [ doi: 10.1016/S0040-4039(02)02271-2 ]synthesis by other group- Mehta G, Islam K.
Total synthesis of the novel angiogenesis inhibitors epoxyquinols A and B.
Tetrahedron Lett. 44, 3569-3572 (2003) [ doi: 10.1016/S0040-4039(03)00621-X ]biological activity- Kamiyama H, Usui T, Uramoto M, Takagi H, Shoji M, Hayashi Y, Kakeya H, Osada H.: Fungal metabolite, epoxyquinol B, crosslinks proteins by epoxy-thiol conjugation.
J Antibiot, 61(2): 94-97 (2008) [ doi: 10.1038/ja.2008.117 ]
[ doi: 10.1038/ja.2008.117 ] - Kakeya H, Onose R, Koshino H, Yoshida A, Kobayashi K, Kageyama S, Osada H.: Epoxyquinol A, a highly functionalized pentaketide dimer with antiangiogenic activity isolated from fungal metabolites.


