ケミカルバイオロジー研究グループ(終了)
RK-682
- Structure
-

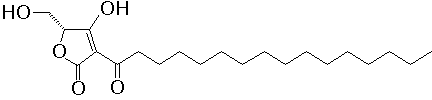
- Producing organism
- Streptomyces sp.
- Biological activity
- protein tyrosine phosphatase inhibitor
- Abstract
-
The mechanism of ATP-induced long-term potentiation was studied pharmacologically using guinea-pig hippocampal slices. Application of 1-10 µM ATP for 10 min transiently depressed and then slowly augmented the synaptic transmission in CA1 neurons leading to long-term potentiation (LTP). This ATP-induced LTP was blocked by the addition of K-252b, an ecto-protein kinase inhibitor, but was enhanced by the addition of RK682, an ecto-phosphatase inhibitor, both of which do not permeate the cell membrane. These results suggest that ATP applied to the perfusate provides enough substrate for ecto-protein kinase to induce LTP through phosphorylation of extracellular domains of membrane proteins in CA1 neurons.
A specific inhibitor of protein tyrosine phosphatase (PTPase), RK-682 (3-hexadecanoyl-5-hydroxymethyl-tetronic acid) was isolated from microbial metabolites. In vitro, RK-682 inhibited dephosphorylation activity of CD45 and VHR with IC50 54 and 2.0 µM, respectively. In situ, sodium orthovanadate and RK-682 enhanced the phosphotyrosine level of Ball-1 cells, a human B cell leukemia, but not the phosphoserine/threonine level. The PTPase inhibitors, however, had the different arrest point on the cell cycle progression. Sodium orthovanadate inhibited the cell cycle progression at G2/M boundary phase, on the other hand, RK-682 inhibited the G1/S transition. - References
-
isolation and structural determination
- Fujii S, Kato H, Furuse H, Ito K, Osada H, Hamaguchi T, Kuroda Y.: The mechanism of ATP-induced long-term potentiation involves extracellular phosphorylation of membrane proteins in guinea-pig hippocampal CA1 neurons.
Neurosci Lett, 187(2): 130-132 (1995)
- Fujii S, Ito K, Osada H, Hamaguchi T, Kuroda Y, Kato H.: Extracellular phosphorylation of membrane protein modifies theta burst-induced long-term potentiation in CA1 neurons of guinea-pig hippocampal slices.
Neurosci Lett, 187(2): 133-136 (1995)
- Hamaguchi T, Sudo T, Osada H.: RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G1phase.
FEBS Lett, 372(1): 54-58 (1995) synthesis and structure-activity relationship
synthesis and structure-activity relationship- Sodeoka M, Sampe R, Kojima S, Baba Y, Morisaki N, Hashimoto Y.: Asymmetric synthesis of a 3-acyltetronic acid derivative, RK-682, and formation of its calcium salt during silica gel column chromatography.
Chem Pharm Bull (Tokyo), 49(2): 206-212 (2001)
- Usui T, Kojima S, Kidokoro S, Ueda K, Osada H, Sodeoka M.: Design and synthesis of a dimeric derivative of RK-682 with increased inhibitory activity against VHR, a dual-specificity ERK phosphatase: implications for the molecular mechanism of the inhibition.
Chem Biol, 8(12): 1209-1220 (2001)
- Fujii S, Kato H, Furuse H, Ito K, Osada H, Hamaguchi T, Kuroda Y.: The mechanism of ATP-induced long-term potentiation involves extracellular phosphorylation of membrane proteins in guinea-pig hippocampal CA1 neurons.


